Title: Is Gilead’s Selonsertib for NASH Dead?
Author: Peter G. Traber, MD
Date: 02/12/2019
Introduction
The search for an effective therapy for NASH with advanced fibrosis/cirrhosis suffered a major setback February 11, 2019 when Gilead Bioscience ($GILD) reported failure of the phase III STELLAR4 trial that evaluated selonsertib in patients with NASH cirrhosis (stage 4 fibrosis). By mid-2019, we expect results of the phase III STELLAR 3 trial that evaluates selonsertib in NASH patients with stage 3 fibrosis. While it is now certainly less likely that STELLAR3 will be positive, as observed by most analysts, we should be careful not to bury selonsertib too quickly.
The STELLAR4 trial enrolled 877 patients into three arms, placebo and selonsertib 6mg and 18mg. After 48 weeks of therapy, the endpoint of at least a 1-stage reduction in fibrosis was achieved in 12.8% of placebo patients, 14.4% of selonsertib 18mg patients, and 12.5% of selonsertib 6mg patients, all non-significant differences. The reduction of at least one stage of fibrosis in NASH cirrhosis patients means one goes from NASH-CRN stage 4 (0-4) down to stage 3 or less. This goal sets a high-bar for any drug with to achieve.
As liver pathology in NASH progresses through the four stages of fibrosis, the amount of collagen accumulation is not linear, as shown in the figure below from a recent conference presentation. In fact, as pathologists always emphasize, equally important as the quantity of collagen (a representative molecule in fibrotic tissue) is the localization of the collagen in the liver lobule. One can see that moving from stage 4, or cirrhosis, to stage 3 or lower represents a large reduction in collagen, much larger than moving from stage 3 to stage 2 or lower. Since the primary interim endpoint for STELLAR3 is the same as STELLAR4, the reduction of at least one stage of fibrosis from stage 3 represents a much less stringent goal than a similar reduction from stage 4 (cirrhosis). Therefore, if there is some anti-fibrotic effect of selonsertib, it is possible that STELLAR3 could meet its primary endpoint, even though STELLAR4 did not.
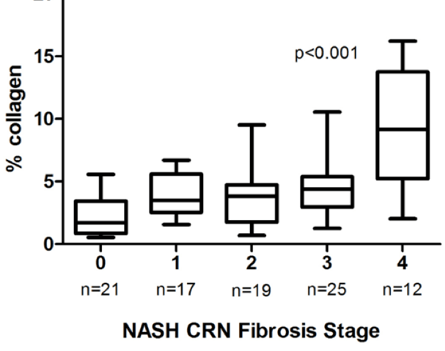
The other point to be recognized from this graph is that it may be an equally small step, in terms of the absolute amount of fibrotic tissue, to progress from stage 3 to cirrhosis (stage 4). The ultimate clinical endpoint for full regulatory approval is reduced progression to cirrhosis. Therefore, it is conceivable that if there is an effect on fibrosis in STELLAR3 that looks promising but does not quite hit the interim endpoint of a one stage reduction in fibrosis, consideration would be given to continuing the trial to evaluate the endpoint of progression to cirrhosis.
The top line data only report the primary endpoint, and a full analysis of the data will be required to understand fully the implications of the study, including patient subsets and other corroborating secondary and exploratory endpoints. While there is likely no resurrecting this phase 3 trial for registration, additional analysis will be important to understand whether there is any potential for selonsertib going forward.
Some observers have suggested that regardless of the results of STELLAR3, there is no chance for approval with a single phase 3 clinical trial. I do not fully agree with this assessment. The target populations of STELLAR3 and STELLAR4 are very different and while it would be ideal if both were positive, a therapy in pre-cirrhotic NASH only would, in my opinion, still be viable. All the patients exposed in both studies would appear to provide the needed size for a safety database, and we will have to wait for a detailed analysis of both studies to determine whether a positive STELLAR3 trial would support an NDA application. While one may speculate on the likelihood of a registration application, one should not rule out the possibility as yet.
Another potential opening for selonsertib, if STELLAR3 is more promising than STELLAR4, is in combination therapy. The ATLAS trial is a phase 2 trial with various combinations of selonsertib, cilofexor (GS-9674) and firsocostat (GS-0976) in NASH patients with advanced fibrosis and cirrhosis that is slated to read out data by the end of 2019.
NASH patients and the medical community will be saddened by a prominent phase 3 trial failure in NASH cirrhosis. However, there is still much to be learned about selonsertib and this signaling pathway in NASH. Moreover, as I wrote in a previous blog, the data from the STELLAR studies when combined with other studies in NASH will provide invaluable data on natural history and refined directions for therapeutic intervention. While the failed STELLAR4 study may have a negative financial impact on $GILD, the company has provided a great service to medical progress by conducting large, well-designed, well-executed, controlled trials and sharing the results through academic channels. Successful drug development is a difficult path made possible by understanding setbacks, learning from all available data, and continually resetting the course.
Additional NASH Insight
- NASH Cirrhosis Space Poised to Heat Up
- Is Rapid Reversal of Liver Fibrosis Possible?
- Industry Strategies for NASH Combination Therapies
- Agencies Clarify NASH Endpoints — But Don’t Harmonize
- NASH Surprises and Interpretation of Clinical Trials
For information on my consulting work please click here.

