Intro:
Over the past two decades, there has been a major shift in the Big Pharma R&D model. In the face of declining efficiencies and increases in both cost and timing for the successful approval and launch of new medicines, large pharma is relying less on internal R&D alone. Discovery collaborations, the in-licensing of early, preclinical, and clinical stage drug candidates, and the acquisition of innovative biotech companies are now central components of pipeline expansion strategies. The aim is mutually beneficial: providing the diversification and added shots-on-target big pharma seeks, while also giving much needed fuel to early-stage innovation. Early partnering provides small biotech and pharma companies with validation and capital for expanding their R&D efforts, enabling the diversification of their pipelines and improving their ability to mitigate risk. Acquisition by large pharma is also an ideal exit strategy for many initial investors.
In 2021, we assembled a list of 64 pharma companies with market capitalizations greater than $10B USD that provided partnering web pages or PDFs on their websites. We then listed and analyzed the data in the paper "What's on Pharma's Licensing Wish List". Given the ever-changing landscape and the resulting shifts in Big Pharma interests, we sought to revisit and revise this analysis and highlight any changes in their appetites over the past two years.
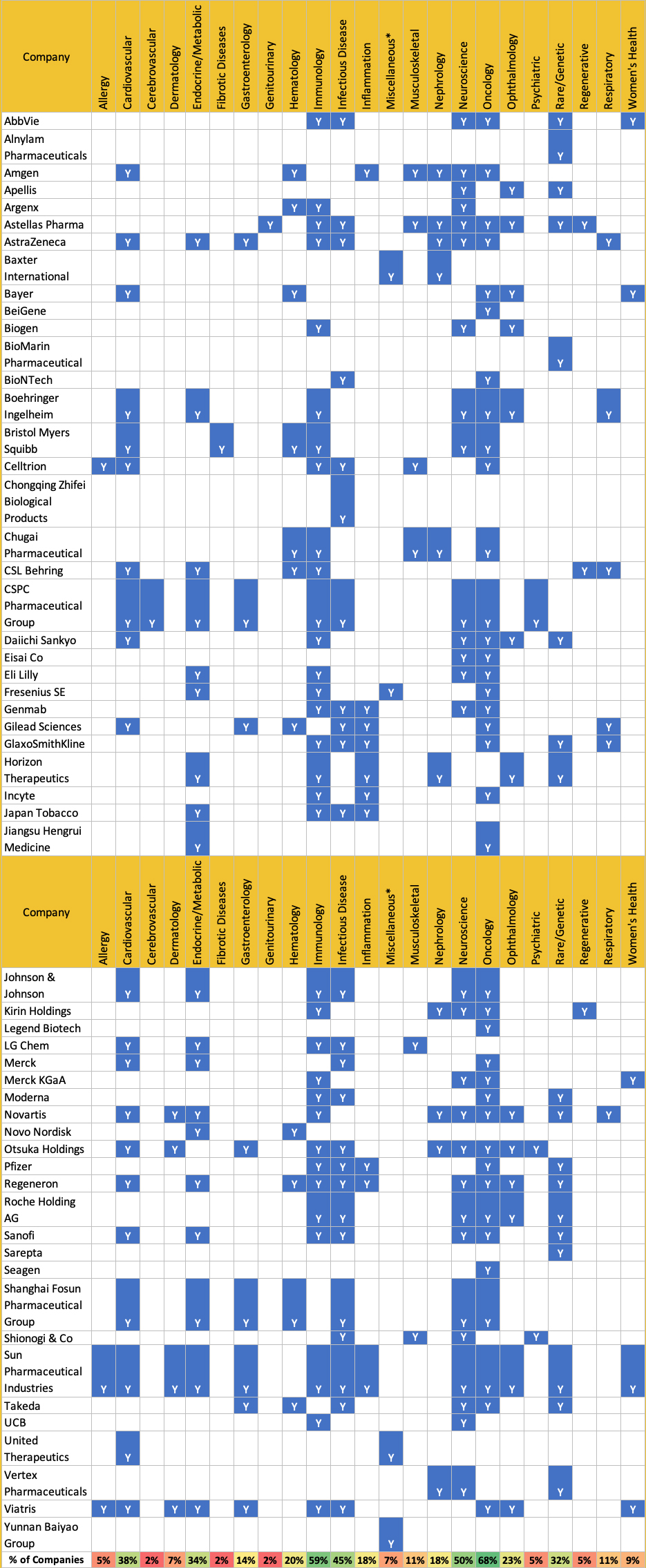
First and foremost, while 56 of the previous companies remain, eight are no longer operating in the same capacity, with some merging and others ceasing operation. For the 56 remaining, in Table 1 we've compiled updated links to each company’s partnering and therapeutic-areas-of-interest webpages, or their downloadable content, and have listed each company’s stated partnering interests as of June 2023. The details these companies provide can indicate either broad descriptions of diseases of interest or more precise details of target indications, modalities, stages of development, and even specific pathways that are considered in scope.
Based on the data compiled, which therapeutic areas appear to have the highest levels of interest?
In order to answer this question, we standardized the areas of partnering interest (Table 2) according to those used by the majority of companies or, when there was no commonly-used specification, we selected a suitable grouping. Using these standardized groupings, there was a broad range in the number of expressed interests by the companies, ranging from one area to thirteen, with the average being 4.8. The most popular areas of partnering interest listed were oncology (68%), immunology (59%), and neuroscience (50%), followed by infectious diseases (45%), cardiovascular (38%), endocrine/metabolic (34%), and rare/genetic (32%). (Figure 1.)
The suggestion here is that a biotech company may have a higher probability of gaining the attention of Big Pharma if its drug development programs target the most sought-after indications. Though, it’s important to note that these companies' published interests may not reflect their actual priorities or actual current and future needs, and as such this information functions best as general guidance rather than as a basis for strategic planning.
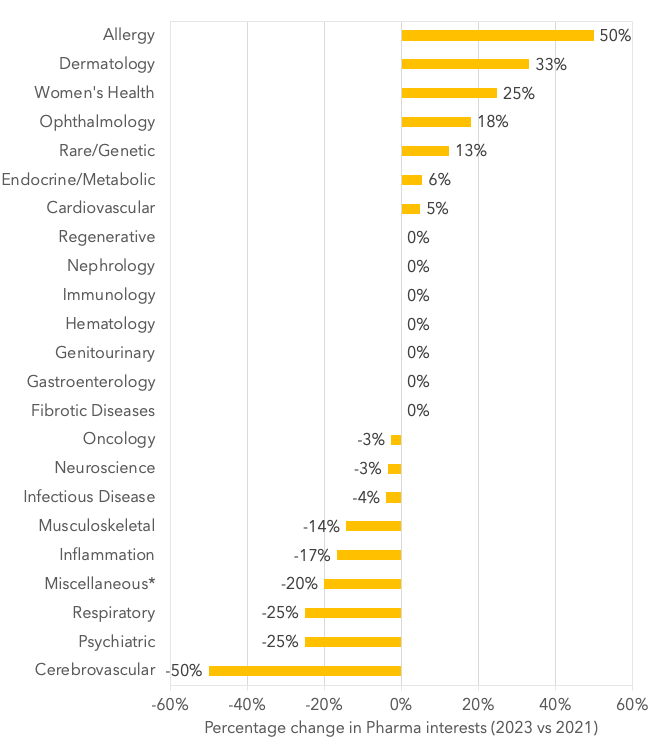
That being said, when we compared the current pharma interests with the interests of those same pharma companies from two years ago (Figure 2), we found increases in the following therapeutic areas:
- Allergy (+50%)
- Dermatology (+33%)
- Women’s Health (+25%)
- Ophthalmology (+18%)
- Endocrine/metabolic: (+6%)
- Cardiovascular: (+5%)
While percentage decreases were observed in the following indications:
- Cerebrovascular (-50%),
- Psychiatric and Respiratory (-25%)
- Inflammation (-17%)
- Musculoskeletal (-14%)
- Infectious disease (-4%)
- Neuroscience and Oncology (-3%)
Lastly, for those interested, a brief partnering primer, can be found at the end of this paper.
Table 1. Pharma Partnering Interests
|
Company |
General Partnering Webpages |
Company Areas of Interest Webpage |
Areas of Interest List |
|
AbbVie |
Immunology, Oncology, Neuroscience, Cystic Fibrosis, Eye care (retinal degeneration, glaucoma, dry eye and ocular surface disease, refractive medicine), Body aesthetics (facial and body filling, breast reconstruction/augmentation, and regenerative soft tissue repair), Women's Health, Virology |
||
|
Alnylam Pharmaceuticals |
https://www.alnylam.com/about-alnylam/investigator-initiated-studies/ |
RNAi therapeutics for genetic diseases, cardio-metabolic diseases, infectious diseases, and central nervous system (CNS) and ocular diseases |
|
|
Amgen |
https://www.amgenbd.com/resource/1610139576000/Brochure_AmgenBD? |
Oncology/Hematology, Cardiovascular Disease, Bone Health, Nephrology, Inflammation, Neuroscience |
|
|
Apellis |
Ophthalmology, Rare Disease, Neurology |
||
|
Argenx |
Autoimmune diseases (myasthenia gravis, immune thrombocytopenia, chronic inflammatory demyelinating neuropathy and pemphigus vulgaris) |
||
|
Astellas Pharma |
Oncology, Urology, Nephrology, Immunology, Neuroscience, Muscle Diseases, ophthalmology, Regenerative Medicine, Vaccines, Gene Therapy |
||
|
AstraZeneca |
Oncology, Cardiovascular, Renal, Metabolism, Respiratory & Immunology, autoimmunity, infection & vaccines, neuroscience and gastroenterology |
||
|
Baxter International |
https://www.baxter.com/our-story/fueling-collaborative-innovation |
https://www.baxter.com/our-story/fueling-collaborative-innovation/baxter-ventures |
In-Hospital Solutions and Therapeutics, Medical devices, Diagnostic tools, Renal therapies |
|
Bayer |
Cardiology, hematology, oncology, women's healthcare, ophthalmology, radiology |
||
|
BeiGene |
https://www.beigene.com/science-and-product-portfolio/research-development |
Cancer (molecular targets), immuno-oncology therapies, combination therapies |
|
|
Biogen |
MS & neuroimmunology, neuromuscular disorders (SMA, ALS), movement disorders (Parkinson's), Alzheimer's & dementia, ophthalmology, neuropsychiatry, immunology, acute neurology, neuropathic pain |
||
|
BioMarin Pharmaceutical |
https://www.biomarin.com/our-company/funding-and-support/independent-research/ |
Genetic therapies for rare diseases |
|
|
BioNTech |
https://servicesplatform.partneringplace.com/OppPortal/portal/BioNTech/ |
https://www.biontech.com/int/en/home/research-and-innovation/therapeutic-areas.html |
Oncology, Infectious Disease (COVID-19, Malaria, Tuberculosis) |
|
Boehringer Ingelheim |
https://www.boehringer-ingelheim.com/partnering/working-together/partnering-interests |
CardioMetabolic Diseases, CNS Diseases, Immunology, Oncology & Cancer Immunology, Respiratory Diseases, Retinal Health |
|
|
Bristol Myers Squibb |
https://www.bms.com/researchers-and-partners/partnering.html |
https://www.bms.com/business-development/science-and-technology-areas-of-interest.html |
Solid tumors, hematology, cardiovascular, immunology, fibrotic diseases, neuroscience, translational medicine, cell therapy |
|
Celltrion |
Biosimilars (cancer, autoimmune disease, Osteoporosis, Asthma, IBS, dyslipidemia), novel antibody drugs (infectious disease, cancer, autoimmune), vaccines, fusion technology |
||
|
Chongqing Zhifei Biological Products |
Vaccines |
||
|
Chugai Pharmaceutical |
https://www.chugai-pharm.co.jp/english/profile/strengths/index.html |
Technology research and genetic analysis for therapeutic antibodies; |
|
|
CSL Behring |
https://www.csl.com/partnering |
Immunology, Hematology & Thrombosis, Transplant, Respiratory, Cardiovascular & Metabolic |
|
|
CSPC Pharmaceutical Group |
Oncology, autoimmunity, psychiatry and neurology, digestion and metabolism, cardio-cerebrovascular system and anti-infectives |
||
|
Daiichi Sankyo |
https://www.daiichisankyo.com/rd/strategy_operations/open_innovation/our_interests/ |
Oncology, rare diseases (monogenic, non-monogenic), CNS, ophthalmology, chronic heart failure, immunology |
|
|
Eisai Co |
https://www.eisai.com/sustainability/partner/partnership/index.html |
Neurology, Oncology |
|
|
Eli Lilly |
Loxo Oncology, neurodegenerative disease, pain & migraine, diabetes and related complications, immunology, new therapeutic modalities |
||
|
Fresenius SE |
https://www.linkedin.com/company/freseniuskabi/ |
I.V. generic drugs, infusion therapies and clinical nutrition products, biosimilars (autoimmune disease, oncology), transfusion technology |
|
|
Genmab |
https://www.genmab.com/research-innovation/antibody-technology-platforms/ |
Antibody therapeutics (cancer, autoimmune conditions, inflammation, central nervous system conditions and infectious diseases) |
|
|
Gilead Sciences |
HIV/AIDS, COVID-19, Liver Disease, Hematology/Oncology, Cardiovascular, Inflammation/Respiratory |
||
|
GlaxoSmithKline |
https://www.gsk.com/en-gb/research-and-development/partnerships/our-areas-of-interest/ |
Respiratory, HIV/Infectious Disease, Vaccines, Oncology, Immune system, Human Genetics, Advanced Technologies |
|
|
Horizon Therapeutics |
https://www.horizontherapeutics.com/science/research-development |
Endocrinology, Nephrology, Neuroimmunology, Ophthalmology, Rare diseases, Rheumatology |
|
|
Incyte |
https://www.incyte.com/what-we-do/collaborate-partners-in-science |
Oncology, Inflammation, Autoimmunity |
|
|
Japan Tobacco |
Glucose and lipid metabolism, immune disorders and inflammation, virus research |
||
|
Jiangsu Hengrui Medicine |
Tumor and diabetes drugs, expand the field of protein and antibody biopharmaceuticals |
||
|
Johnson & Johnson |
Cardiovascular & Metabolism; Immunology; Infectious Diseases & Vaccines; Neuroscience; Oncology; and Pulmonary Hypertension |
||
|
Kirin Holdings |
https://www.kyowakirin.com/what_we_do/research_collaboration_licensing/index.html |
Therapeutic antibodies (oncology, nephrology, central nervous system and immunology), small molecule drugs, nucleic acid drugs, regenerative medicine |
|
|
Legend Biotech |
Oncology (hematologic malignancies and solid tumors) |
||
|
LG Chem |
https://www.lgchem.com/upload/file/introduce/2021_Introduction_of_LGChem_EN[15].pdf |
Has Life Science division with products in diabetes, cardiovascular, musculoskeletal, autoimmune, growth hormones, hepatitis B and pentavalent vaccines, aesthetics |
|
|
Merck |
Oncology, Vaccines, Infectious Diseases, COVID-19, cardio-metabolic disorders |
||
|
Merck KGaA |
https://www.emdgroup.com/en/company/partnering/collaboration/collaboration-in-healthcare.html |
Oncology, Immuno-Oncology, Immunology, Multiple Sclerosis, Fertility |
|
|
Moderna |
https://www.modernatx.com/partnerships/strategic-collaborators |
https://www.modernatx.com/research/therapeutic-areas |
infectious diseases, immuno-oncology, rare diseases, cardiovascular diseases and autoimmune diseases |
|
Novartis |
Oncology, Cardiovascular, Metabolic & Renal Diseases, Immunology & Dermatology, Neuroscience, Ophthalmology, Respiratory Diseases, Cell and Gene Therapy |
||
|
Novo Nordisk |
https://www.novonordisk.com/research-and-development/partner-with-us.html |
Type 1 Diabetes, Type 2 Diabetes, obesity, growth disorders, hemophilia |
|
|
Otsuka Holdings |
https://www.otsuka.co.jp/en/partnering-and-licensing/inquiry-form/form |
Neuroscience, nephrology, autoimmune, oncology, gastrointestinal disorders, cardiovascular diseases, ophthalmology, dermatology |
|
|
Pfizer |
Internal Medicine, Rare Disease, Inflammation & Immunology, Vaccines, Oncology |
||
|
Regeneron |
Cardiovascular/Metabolic diseases, Infectious diseases, Inflammation and Immunology, Oncology, Hematology, Ophthalmology, Neurology, Rare Diseases |
||
|
Roche Holding AG |
https://www.roche.com/partnering/about_partnering_at_roche.htm |
https://www.roche.com/partnering/pharma-areas-of-interest.htm |
Oncology and cancer immunotherapy, immunology, infectious diseases, neuroscience, ophthalmology, rare diseases |
|
Sanofi |
https://www.sanofi.com/en/partnering |
Immunology, Oncology, Neuroscience, Rare Diseases, Vaccines, Diabetes, Cardiovascular |
|
|
Sarepta |
https://www.sarepta.com/science/strategic-partners |
https://www.sarepta.com/disease-areas |
Rare genetic diseases (Duchenne muscular dystrophy, limb-girdle muscular dystrophies, and Charcot-Marie-Tooth disease) |
|
Seagen |
https://www.seagen.com/patients-and-caregivers/disease-we-study |
Hodgkin lymphoma, cutaneous and peripheral T-cell lymphoma (CTCL, PTCL), cancer (breast, lung, bladder, ovarian) |
|
|
Shanghai Fosun Pharmaceutical Group |
Metabolism and digestive tract, anti-tumor, anti-infective, CNS, cardiovascular, blood diseases, vaccine |
||
|
Shionogi & Co |
https://www.shionogi.com/global/en/innovation/partnering.html |
https://www.shionogi.com/global/en/innovation/randd/drug-discovery/what-we-are-focusing-on.html |
Infectious disease, pain CNC (novel mechanisms), novel modalities |
|
Sun Pharmaceutical Industries |
CNS, Cardiovascular, Women Health, Dermatology, Ophthalmology, Oncology, Anti-Infectives and Gastro therapies, orphan indications |
||
|
Takeda |
Oncology, Rare Diseases, Neuroscience, and Gastroenterology (GI), Plasma-Derived Therapies, Vaccines |
||
|
UCB |
Neurology, immunology, rare diseases |
||
|
United Therapeutics |
https://www.unither.com/contact |
https://pipeline.unither.com/ |
Pulmonary arterial hypertension, Pulmonary hypertension, and End-stage organ disease |
|
Vertex Pharmaceuticals |
https://www.vrtx.com/we-are-vertex/collaborations-partnering |
https://www.vrtx.com/we-are-vertex/collaborations-partnering#areas-of-interest |
Cystic fibrosis, Alpha-1 Antitrypsin Deficiency (AATD, Sickle Cell Disease and Beta Thalassemia, Pain, APOL1-Mediated Kidney Disease, new platform technologies that will enable exploration of new diseases or disease targets |
|
Viatris |
https://www.viatris.com/en/about-us/corporate-social-responsibility/partnerships |
https://www.viatris.com/en/products/therapeutic-areas |
Cardiovascular, CNS & Anesthesia, Dermatology, Diabetes & Metabolism, Eye Care, Gastroenterology, Immunology, Infectious Disease, Oncology, Respiratory & Allergy, Women's Healthcare |
|
Yunnan Baiyao Group |
Chinese patent medicines, Chinese traditional medicines, biological products, health food, cosmetics and beverages, and medical devices |
Table 2. Standardized pharma partnering interests
*Miscellaneous includes hospital, generics, internal medicine, orthopedics, medical devices, Chinese traditional medicine
Figure 1. Percentage of companies expressing interest in standardized therapeutic area.
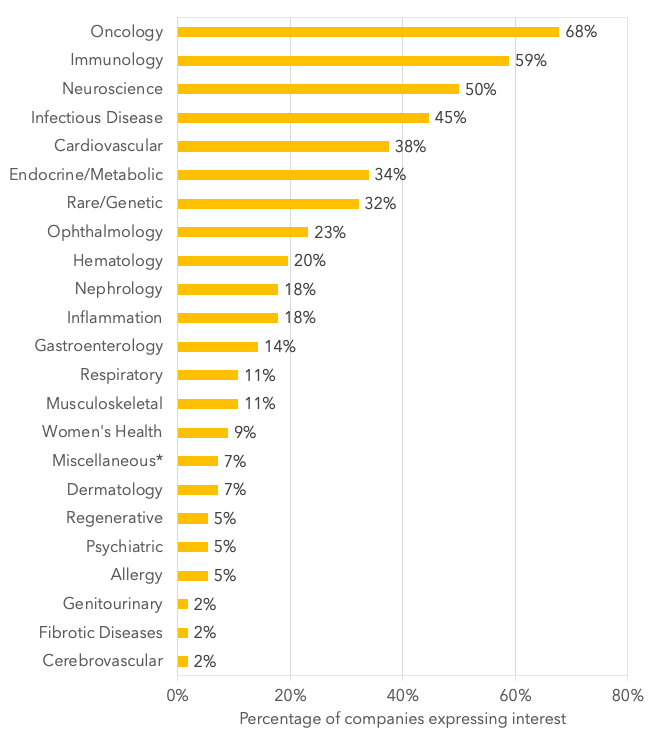
Figure 2. Percentage change in companies expressing an interest in standardized therapeutic areas (2023 vs 2021).
Brief Partnering Primer
Whether or not your company is developing a therapeutic for a ‘hot’ indication or drug target, it is crucial to be prepared in advance of reaching out to potential partners. The entire partnering process for a pharmaceutical asset can take over a year to complete, depending on a number of factors such as the type of molecule and disease indication, how advanced the program is in development, what volume of data needs to be considered, how competitive the space is, and how complex the deal and contract terms are. Alacrita has considerable experience in supporting companies with partnering and can provide assistance across any or all components of the partnering process.
To ensure your asset is ready for partnering, we recommend the following initial workplan:
- Build a virtual data room
- Mock due diligence and gap analysis
- Prepare non-confidential (NC) and confidential pitch decks
- Identify and prioritize prospective partners for outreach
Alacrita can assist you with these steps, and our network of expert consultants ensures we have the scientific and business expertise on hand to highlight the strengths of your asset and express its value. If you are already in discussions with potential partners, we can provide you with a deal comparables analysis, sales forecast and rNPV asset valuation, and/or transactional support to ensure that deal terms are fair. Alternatively, if your company is in a position to in-license an asset, our team of asset scouting experts can help you source and evaluate assets that meet your search criteria and enable you to diversify your pipeline.
Pharma out-licensing, partnering, & deal-making support
We work with clients to ensure that partnership, licensing and investment decisions are taken with confidence, with clear alignment between transaction price and asset value. We also help many clients prepare for the partnering process, including with conducting partnerability assessments.

