Introduction:
Anti-fibrotic drug candidates, prior to entering clinical development, often have a mechanism of action that may be beneficial for treatment of multiple diseases. Early strategic issues in advancement of such drugs include identification of disease indications with the best likelihood of success in proof of concept (POC) and registration clinical trials and predicting the economic impact on the company for various development pathways.
All tissues and organs contain connective tissue, including various types of collagen, elastin, and others, that provides architectural support for parenchymal cells. The amount of connective tissue is normally finely tuned so that a steady-state is balanced between production and degradation. In response to various inflammatory or toxic insults production of collagen increases more than degradation leading to accumulation of fibrotic tissue. This is a normal wound healing response to acute insults. However, when the insult is chronic, fibrogenesis may lead to pathologic accumulation of fibrotic tissue (scarring) which may result in organ dysfunction and failure.
Fibrosis is the final common pathway for most pathophysiological processes in multiple organs and is the primary reason for organ transplants. In fact, it has been estimated that nearly 50% of deaths in the developed world is due to fibrosis of key organs including heart, liver, kidney, lung, and others. Therefore, pharmaceutical and biotech companies are keenly focused on developing drugs that can prevent, mitigate or reverse the fibrotic process.
Fibrotic Conditions are Many and Diverse:
There are many fibrotic conditions, but here is a list of some of the best known:
Pulmonary Fibrosis:
- Idiopathic Pulmonary Fibrosis (IPF): The most common type of pulmonary fibrosis, with no known cause.
- Cryptogenic Organizing Pneumonia (COP): A rare form of pulmonary fibrosis characterized by inflammation and fibrosis in the small airways and alveoli.
- Disease-Related Pulmonary Fibrosis: Caused by underlying diseases such as autoimmune conditions (e.g., rheumatoid arthritis, scleroderma), viral illnesses, or gastroesophageal reflux disease (GERD).
- Hypersensitivity Pneumonitis: An immune-mediated lung disease that can lead to fibrosis.
- Asbestosis: Caused by exposure to asbestos fibers, resulting in lung scarring.
- Silicosis: Occurs due to inhalation of silica dust, leading to lung fibrosis.
Skin Fibrosis:
- Scleroderma (Systemic Sclerosis): A connective tissue disorder characterized by fibrosis affecting the skin, blood vessels, and internal organs.
- Morphea: Localized skin fibrosis that presents as thickened, discolored patches.
- Keloids: Abnormal scar tissue formation after skin injury.
Liver Fibrosis:
- Liver Cirrhosis: Chronic liver disease characterized by extensive fibrosis and impaired liver function.
- Non-Alcoholic Steatohepatitis (NASH): Liver fibrosis related to non-alcoholic fatty liver disease.
- Primary Biliary Cholangitis (PBC): Autoimmune condition leading to bile duct inflammation and fibrosis.
Renal Fibrosis:
- Chronic Kidney Disease (CKD): Progressive kidney damage resulting in fibrosis and impaired function.
- IgA Nephropathy: Immune complex deposition in the kidney leading to fibrosis.
- Alport Syndrome: Genetic disorder causing kidney fibrosis and hearing loss.
Cardiac Fibrosis:
- Myocardial Infarction (Heart Attack): Scar tissue formation in the heart muscle after a heart attack.
- Hypertrophic Cardiomyopathy: Thickening of heart muscle fibers due to fibrosis.
- Restrictive Cardiomyopathy: Stiffening of heart chambers caused by fibrosis.
Gastrointestinal Fibrosis:
- Crohn's Disease: Chronic inflammation in the gastrointestinal tract leading to fibrosis.
- Intestinal Strictures: Narrowing of the intestine due to fibrotic scarring.
- Radiation Enteritis: Fibrosis following radiation therapy.
Other Fibrotic Conditions:
- Myelofibrosis (MF): A rare blood cancer where scar tissue forms in the bone marrow, disrupting normal blood cell production.
- Dupuytren's Contracture: Hand deformity caused by palmar fascia fibrosis.
- Peyronie's Disease: Penile fibrosis resulting in curvature during erection.
- Endomyocardial Fibrosis: Rare heart condition affecting the endocardium.
- Retroperitoneal Fibrosis: Fibrosis around abdominal structures.
- Nodular Fasciitis: Benign fibrous tumor affecting soft tissues.
General Principles of Indication Selection:
Many people like to score indications against a relevant range of parameters (including, e.g., applicability of the mechanism of action, unmet need, available population size, time/cost to clinical proof of concept (POC), pricing potential, competitive intensity, etc.) and then calculate a composite, sometimes weighted, overall score to generate a rank-ordered list of priorities. As we have written before (https://www.linkedin.com/feed/update/urn:li:activity:7100271597042774016), this approach is fundamentally flawed. Five scores of 4 out of five is much better than four 5s and a zero. Numerically, they both sum (unweighted) to 20, but the second opportunity has a zero which generally implies that the indication in question is simply not feasible due to that particular parameter.
Root Cause Analysis Presents Challenges
Although chronic inflammation commonly leads to fibrosis, the underlying causes are often poorly understood and disease etiology is not well characterized. There are some exceptions, e.g., viral hepatitis C, but the advent of highly effective antivirals has for the most part obviated this condition where healthcare systems and resources are adequate. At the other end of the spectrum are diseases such as primary sclerosing cholangitis (PSC) where the current state of play makes even the agreement on clinically-relevant trial endpoints a source of contention. It is toward such conditions that anti-fibrotics have been aimed absent the ability to target the primary causal factor. This includes diabetes-associated fibrosis, especially of the kidney, where the current glucose control measures have not proven sufficient to prevent the fibrotic complications.
Over the past decade, large indications such as NASH, associated with or driven by the obesity epidemic, have received outsize attention with dramatic rises and falls of the share prices of developers. Most recently (Feb 2024), Madrigal published Phase 3 NASH data and although positive, an accompanying editorial in the NEJM stated “although resmetirom treatment was successful, the placebo-subtracted effect of resmetirom was overall modest (16.4 to 20.7 percentage points for NASH resolution and 10.2 to 11.8 percentage points for fibrosis), which means that approximately 2 of 10 patients treated will have NASH resolution and approximately 1 of 10 patients treated will have fibrosis improvement”. At almost the same time, Lilly released Phase 2 data in biopsy-proven NASH patents showing that tirzepatide, the GLP-1 drug for diabetes and obesity, resulted in a decrease in fibrosis by at least one stage with no worsening of MASH on liver histology. According to Stifel analysis, “at first blush, the Lilly TZP data look far better than what is seen with [89bio’s pegozafermin, Intercept’s ocalivia, Madrigal’s resmetirom, Inventiva’s lanifibranor and Akero’s efruxifermin]. Indirect antifibrotics appear to us to have met their match.” The partial efficacy of these disparate drugs highlights the challenges in understanding which mechanistic basis to apply to address the etiology of the disease.
Drug Mechanism of Action (MOA) Considerations:
Anti-fibrotic drugs often have many potential indications because they target molecules and pathways involved in fibrosis of multiple organs. Additionally, some targets may affect several pathologic pathways in addition to purely anti-fibrotic mechanisms including, immune cell modulation, intermediary metabolism, cytokine expression, and cell death. Consideration of potential indications is greatly aided by deep understanding of the disease pathophysiology and how the drug may affect multiple pathways. This is also important for potential disease biomarkers, discussed below. Close interaction between scientific and development teams is critical during the early stages of selecting therapeutic indications.
One of the important considerations in pre-clinical pharmacology experiments is whether the drug inhibits the development or progression of fibrosis or whether it reverses existing fibrotic tissue, or ideally both. While the design of pre-clinical experiments to ascertain this may be challenging, such an effort will be rewarded in designing the most effective development program.
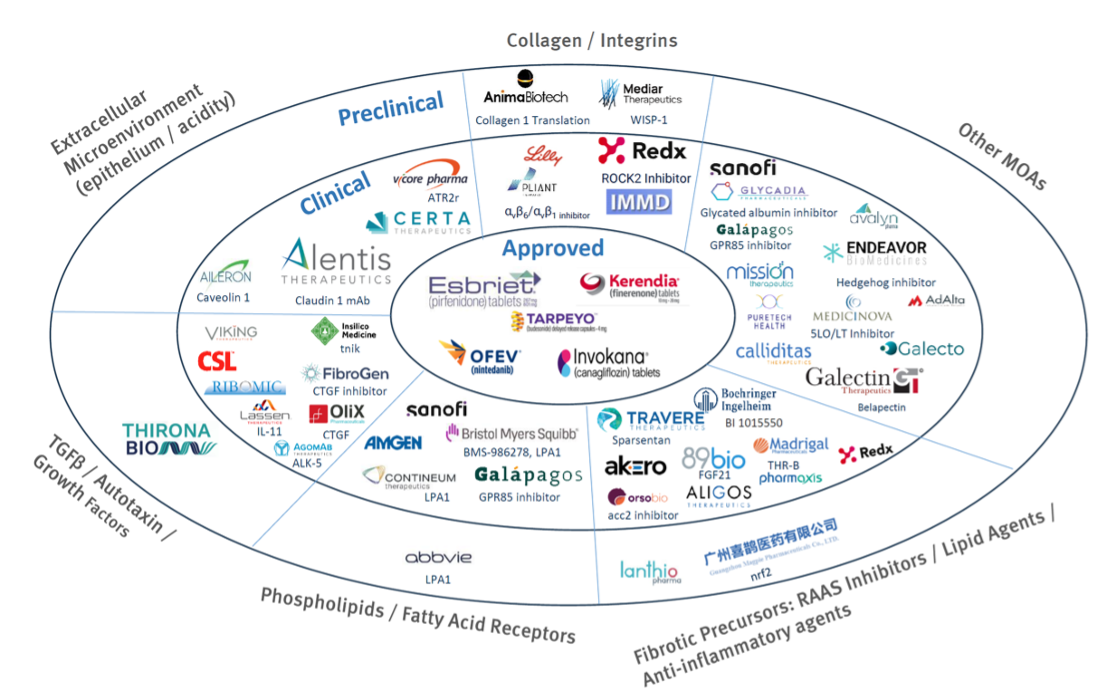
The following graphic (Source: Stifel Research and DealForma) illustrates a non-comprehensive selection of the current fibrosis pipeline by mechanism of action. As is the case with many other complex etiologies, we believe it is likely that a combination of modalities will be required for optimum efficacy rather than any single silver bullet.
Medical Considerations:
One of the highest hurdles to clinical development is that fibrosis usually progresses slowly and, as a corollary, clinically meaningful therapeutic changes may take a long time to manifest. This is clearly incompatible with biotech timeframes forcing the need to use biomarkers or surrogate endpoints. The problem here is that validated parameters are few and far between. Given that target cells in fibrotic disease are often embedded in thick layers of fibrotic tissue, simple blood markers may prove insufficient to reflect underlying target engagement or therapeutic activity. The holy grail for a new therapeutic agent is to reverse fibrosis, but in many settings this is considered an exceptionally high bar, but the alternative, namely slowing or halting progression of fibrosis, is hard to measure given the variability in kinetics of disease between patients. Nevertheless, recent data are promising, e.g., pelabresib (Morphosis) induced a reduction in bone marrow fibrosis in a significant proportion of primary myelofibrosis patients, and it could be argued that such performance is truly necessary to generate a meaningful clinical impact.
For any new agent, there should a clear pathway to POC as well as the subsequent studies necessary for approval, which may include provisional approval based on an endpoint that is a surrogate for clinical outcomes. However, in many situations the existing endpoints are not viewed by regulatory agencies as validated surrogates nor sufficient clinical endpoints for approval of the indication. Whatever the planned clinical endpoints, preclinical experiments should attempt to evaluate these endpoints when feasible to provide some confidence of the success of POC clinical trials.
Assessment of endpoints that regulatory agencies consider validated or potential surrogates and approvable endpoints is essential. Such an assessment may be straightforward in the situation where there are already approved drugs for the indication. For example, in idiopathic pulmonary fibrosis (IPF), there are two marketed drugs (pirfenidone and nintedanib), one of which is an anti-fibrotic agent (pirfenidone) that inhibits TGF-β induced collagen deposition. In this case, approvable endpoints are well-defined, although the sequencing of endpoints in clinical trials for POC and evaluation of additional potential surrogates may need additional thought depending on the drug MOA.
A common situation with anti-fibrotic drugs is there are no approved drugs for the indication and a lack of clarity from regulatory agencies on appropriate endpoints. One pitfall companies may encounter is that regulatory agencies are not prepared to commit to trial endpoints even after clinical trials are well underway. The early years in fibrosis drug development for specific indications is a process of data analysis and discussion between industry, regulatory agencies, and academia before appropriate endpoints are agreed. This can be a trying process for companies in the early years, but those that enter later benefit from the previous activity even if there are no approved therapies.
Conclusions:

As shown in the above (non-comprehensive) graphic (Stifel Research and DealForma), there is a reasonable pipeline of anti-fibrotic drugs that are already committed to specific indications. Many of these are likely to provide supportive care/progression reduction only, and few will be truly disease-modifying. Any new anti-fibrosis candidate will need to be carefully targeted by matching disease etiology with mechanism of action and set in the context of clinical trial/endpoint feasibility.
About the Authors:
- Anthony Walker, PhD, Managing Partner, Alacrita Consulting
Anthony leverages more than 35 years of experience, including over a decade spent building & managing a biotechnology company and over 20 years as a management consultant to the pharmaceutical & biotech industries. - With contributions from Peter Traber, MD, PhD.
Fibrosis Consulting Support:
We support clients on a range of drug development products and programs targeting fibrosis, and have advised healthcare investors and business development teams on individual assets, pipelines and companies in this promising space.
Our core team draws from an extensive consulting network, which has close to 40 senior consultants experienced with anti-fibrotic therapeutics. These specialists understand the intricate complexities and unique challenges present in their development and commercialization and can help you effectively navigate through them. Our extensive resources in this field allow us to build project teams that precisely match the expertise needs of your project.

