Interim Chief Medical Officer Support
Alacrita has a number of CMO-level clinical MDs in our consulting group, who support our biotech clients as interim Chief Medical Officers, during for example, periods of extended search for a full-time individual. These scientific leaders are capable of overseeing flagship clinical trials, guiding a portfolio of programs, providing leadership to junior medics in the organization, as well as representing the company with the board of directors, investors and regulatory authorities. Our CMO consultants are typically available for as much time as the engagement requires.
Our interim Chief Medial Officers provide a range of support, including:
- Overseeing clinical trials.
- Developing and/or leading clinical development roadmaps, including clinical trial strategy and execution plans.
- Providing strategic and hands-on regulatory support, including handling regulatory interactions and responses, and ensuring the successful filling of INDs.
- Providing guidance and budgetary oversight for clinical operations, medical affairs, and patient advocacy.
- Providing strategic insight into the competitive landscape of your clinical programs and pipeline assets.
- Providing clinical and scientific input into discovery, pre-clinical development and early pipeline activities, from target selection to preclinical and regulatory strategy.
- Providing medical and scientific expertise to the organization, executive team and board of directors
- Representing the company in meetings and communications with external stakeholders including investors and partner organizations.
- Supporting fundraising activities.
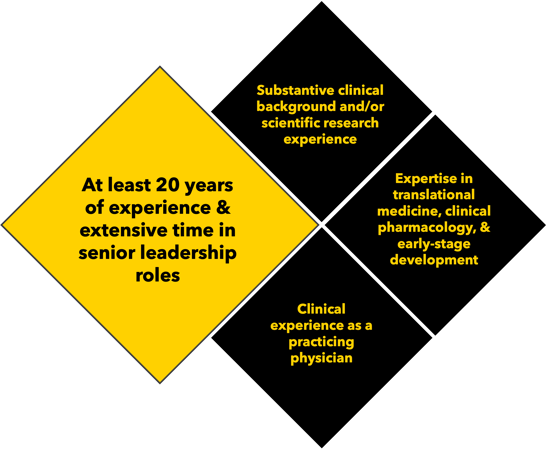
Each of our Chief Medical Officer consultants meets the following criteria:
- At least 20 years of experience, with significant time spent in senior leadership roles.
- Substantive clinical background and/or scientific research experience in the technology and disease area of your program.
- Clinical experience as a practicing physician.
- Expertise in translational medicine, clinical pharmacology, and early-stage development.
Featured Interim CMO Support Case Studies:
Challenge: Alacrita provided interim chief medical officer support to an oncology company over the course of two years. The company was a developing a portfolio of first in class small molecules to treat a range of tumor types. Three of the products were in Phase I clinical trials, the first and most advanced was recruiting patients in a range of hematologic malignancies including acute myeloid leukemia, myeloid dysplastic syndrome and chronic lymphocytic leukemia. The second and third molecules were subject of Phase I/II trials in specific solid tumors. The company was well-capitalized with around 80 employees. The programs were discovered internally by the company’s cancer research efforts and were spearheading the company’s transition from a research-oriented biotech to a company focused on clinical development. The molecules, which targeted new cancer biology, were potent and selective and targeted at genomically defined subsets of cancer patients. The company was developing and implementing a biomarker strategy for each asset.
Solution: Alacrita placed a board-certified oncologist with several years of drug development experience in the biotech environment as interim chief medical officer in the company. He spent significant time on-site per week at the client’s premises and embedded himself into the team to ensure a high impact engagement. Having ultimate responsibility for the clinical team at the company, he had diverse responsibilities. Key achievements and responsibilities included: led the oncology clinical team, helping recruit and retain additional medical talent, advised the CEO on strategic clinical decisions, represented the company at investor, partnering and investigator meetings.
Challenge: A listed biotech company conducting Phase III clinical trials in an oncology indication needed Chief Medical Officer support for multiple clinical activities in Europe and the United States.
Solution: We provided medical oversight of three ongoing clinical trials, in particular, providing medical oversight for a Phase I trial and preparing for:
- Marketing application
- Data and safety measurement board (DSMB) review of an ongoing Phase IIb clinical trial
- Launch of a Phase II clinical study in pancreatic cancer
Challenge: Following an engagement involving defining a clinical strategy for an innovative biopharma company, an experienced Alacrita partner was deployed as Interim CMO at an innovative biopharma client for over two years.
Solution:
Key activities included:
- Continued development of the lead candidate drug and other therapies in the company’s portfolio
- Medical support for the associated regulatory filings
- Representation of the company to investors and other external parties.
Challenge: A privately-held European biopharma company focused on immuno-oncology therapeutics, in collaboration with academic investigators and inventors, was developing small molecule and monoclonal antibody agents for three targets of high interest in the I/O field. The company had a sudden departure of their Chief Medical Officer and needed interim support while recruiting for a permanent replacement. Alacrita was engaged to provide that expertise.
Solution: Alacrita provided Chief Medical Officer support for the client, and assisted on several activities such as:- Provided clinical development input on the design of a FIH trial and clinical development plan for a differentiated small molecule that targeted a receptor of high interest in the I/O field. This study included dose-escalation for the novel agent as monotherapy and in combination with the anti-PD-1 antibody Keytruda (pembrolizumab). The agent was being developed in collaboration with a non-profit cancer research organization. The phase 1 trial was to be conducted at a prominent U.S. academic center. Alacrita contributed to joint team meetings regarding the FIH trial and IND preparation as well as meetings with the KOL-lead investigator;- Provided input on the clinical development plan and FIH study design for the next-most advanced agent in the company’s portfolio; - Joined the company’s executive team members for a meeting with U.S. venture investors during due diligence, which led to a successful Series B financing;- Assisted in evaluating and interviewing candidates for the permanent CMO position.

