Immunology Consulting Support
We support clients on a range of immunology drug development products and programs, and advise healthcare investors and business development teams on individual assets, pipelines and companies in this promising space. Our core team draws from an extended consulting network, which has over 120 senior consultants experienced with immunology therapeutics. These specialists understand the unique and complex set of challenges present in their development and commercialization and can help you effectively navigate through them. Our extensive resources in this space allow us to field project teams that precisely match the expertise needs of each project.
The support we can provide ranges from opportunity mapping and business strategy to regulatory affairs, preclinical and clinical support, to due diligence, valuations and licensing, partnering & dealmaking, among other consulting service.
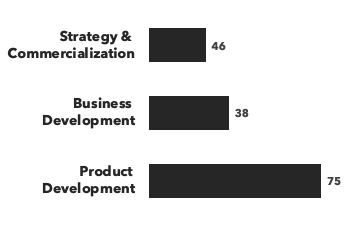
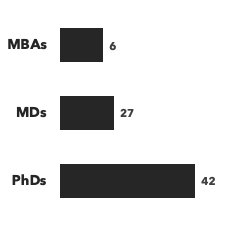
Number of consultants with immunology expertise, by discipline and by education:
Recent CNS Consulting Projects:
- Providing due diligence in inflammation and fibrosis area: An innovation interested in a research institute developing proprietary mAbs against a novel liver disease target, asked Alacrita to conduct due diligence on the development of this novel target. The institute had identified a novel chemokine pathway that mediates inflammation and fibrosis, and has developed proprietary antibodies to inhibit this pathway.
- Crohn's Disease research project plan: A research group at a leading university had manipulated autologous regulatory T cells (Tregs) to give them gut-homing properties, making them suitable for influencing the course of inflammatory bowel diseases such as Crohn’s disease and ulcerative colitis. The commercial potential for this novel treatment was thought to be substantial if the logistic aspects of harvesting and delivering the autologous cellular therapeutic product could be addressed. Alacrita was asked to produce a commercial development plan to map out the opportunity and value the research program.
- Providing scientific and immunological mechanism of action due diligence for a big pharma client: A major pharma company needed scientific and immunology due diligence support on a novel cyclic peptide, targeting the complement cascade system. We reviewed the scientific and mechanistic experiments underpinning the clinical program to understand the risk around a clinical study failing an efficacy endpoint. The research program and resultant data demonstrated a compelling mechanistic rationale to treat the disease and we recommend that our client should proceed from the standpoint of an immunological mechanism of action and scientific merit.
- Pipeline strategy for gene therapy company: A leading biotechnology company with a focus on development and commercialization of gene therapies for bleeding and other debilitating disorders had developed a next-generation AAV gene therapy platform. The company wanted to build its pipeline with other drugs against autoimmune and complement-mediated diseases. Along with support from investors, they were in the process of evaluating a long list of potential indications in terms of technical feasibility with their platform technology, and approached Alacrita to support additional appraisal of short-listed indications for prioritization.
- Commercial development plan for a mesenchymal stem cell therapy in acute GvHD: Our client was a leading UK Research Institute requiring assistance in assigning internal commercialization funding to the most promising research groups within the university. Alacrita was asked to assess the viability and market attractiveness of selected projects and to develop individual Commercial Development Plans (CDP) that could be reviewed by the funding committee. One such group requiring a CDP was developing a new mesenchymal stem cell (MSC) therapeutic for the treatment of acute graft versus host disease.
- Providing CTA submission support for a monocloncal antibody in Primary Sclerosing Cholangitis: Our client specialized in the development of proprietary monoclonal antibodies directed towards novel targets for the treatment of immune-mediated and fibrotic disorders, including orphan indications. It had identified a novel pathway that mediated inflammation and fibrosis, and as such had developed proprietary antibodies to target it. For the lead antibody the client planned to initiate a Phase IIa proof of concept (POC) repeat dose safety study in patients with a fibrotic liver indication and asked Alacrita to help create the IMPD and manage the submission of the Clinical Trial Application and associated approvals.
- T cell therapy commercialization plan: The commercialization arm of a leading university wished to identify its most promising therapeutic programs within its purview in order to allocate internal translational funding to progress into the clinic. Once a subset of research groups had been selected by the commercialization team, Alacrita was commissioned to create Commercial Development Plans for each program which would be presented to the funding board. One of the programs selected was a Treg cell therapy in preclinical development for the treatment of drug-refractory immune conditions of the liver. The product had potential not only to stop disease progression but was also proposed to provide regenerative effects.
- Preclinical gene therapy development support: A biotech company developing novel gene therapy approaches for in vivo cell trans-differentiation required an AAV gene therapy expert to serve on a scientific advisory board (SAB) tasked with ongoing analysis and development planning of preclinical studies for two lead programs.
Selection of Immunology Consulting Case Studies
Challenge: The commercialisation arm of a leading university wished to identify its most promising therapeutic programs within its purview in order to allocate internal translational funding to progress into the clinic. Once a subset of research groups had been selected by the commercialisation team, Alacrita was commissioned to create Commercial Development Plans for each program which would be presented to the funding board. One of the programs selected was a Treg cell therapy in preclinical development for the treatment of drug-refractory immune conditions of the liver. The product had potential not only to stop disease progression but was also proposed to provide regenerative effects.
Solution: Alacrita met with the research team to discuss the project in more detail, before reviewing all available material and creating the Commercial Development Plan. This plan outlined the potential value in the program and the technical proof of concept milestones that the group had to reach in order to progress their product into first-in-human studies.
Specifically, the document analysed and outlined the following:
- Background on the technology/invention;
- Current stage of development; existing proof of concept data;
- Development plans;
- Competence of the project team;
- Intellectual property;
- Unmet medical needs addressed by the program;
- Market opportunity;
- Competitor landscape; and
- Key risks in the project and steps to mitigate them.
Our report was discussed with the commercialisation and research team before a final version was sent to the internal funding committee for review.
Challenge: A biopharma company required expert due diligence for a potential transaction with a clinical stage biotech focusing in inflammation and fibrosis. Alacrita was commissioned to provide an assessment of the target company's lead clinical candidate, a first-in-class monoclonal antibody in development for multiple autoimmune and liver diseases.
Solution: Alacrita reviewed all relevant company information, including preclinical publications, pharmacology and toxicology reports, clinical trial documentation and plans, CMC strategy, regulatory correspondence and IP. Together with an evaluation of the competitive landscape and market opportunity, our expert team quantified the commercial and technical risks associated with the investment. This was presented to the client as a detailed report summarizing the marketable advantages of the lead molecule with important risk areas flagged and ranked. Our client proceeded with the investment and within two years, the company was listed on NASDAQ.
Challenge: Our client was a leading UK Research Institute requiring assistance in assigning internal commercialisation funding to the most promising research groups within the university. Alacrita was asked to assess the viability and market attractiveness of selected projects and to develop individual Commercial Development Plans (CDP) that could be reviewed by the funding committee. One such group requiring a CDP was developing a new mesenchymal stem cell (MSC) therapeutic for the treatment of acute graft versus host disease.
Solution: Alacrita's specialist stem cell consultant met with the Principal Investigator to collect information on all areas of the project and from this created a CDP covering the following key areas:
- Background on the project and research team
- Rationale of the therapeutic approach
- Current unmet clinical need
- Clinical differentiation of the therapy
- Competitive landscape
- Pathway to commercialisation
- Development milestones
- Budget for the project.
The CDP was reviewed by the funding commitee and the project was successful in achieving funding.

