Looking Beyond GLP-1s
Currently, much of the pharmaceutical industry and up to a quarter of the population of the developed world are captivated by get-slim-quick drugs known as GLP-1 agonists, and variations thereof. There appears to be no questioning their efficacy, certainly not as reflected by the extraordinary level of self-pay sales dwarfing all other therapeutic categories. These therapeutics with a seemingly vast market potential are the current darlings of the space, but they are from the end all be all. This paper explores the field beyond GLP-1 and looks for signals to other therapeutic approaches that may emerge in the coming years.
Obesity is associated with several significant complications as well as increased cardiovascular risk, particularly regarding abdominal obesity characterized by the accumulation of visceral fat. This type of obesity typically aligns with the criteria for metabolic syndrome, requiring at least three of the following: elevated waist circumference, elevated triglycerides, reduced high-density lipoprotein (HDL) cholesterol, hypertension, and elevated fasting glucose. The use of the term obesity in this paper includes any or all of these facets as it is not intended to be a comprehensive medical review.
Our starting point is far from the mainstream and may be surprising: uric acid, the metabolite which, in excess, causes gout. It has been characterized as evolving from a passive participant in metabolic syndrome to an active participant. We then explore the role of inflammation (also linked to uric acid) and onto effects on the central nervous system and behavior. We end with a brief look at the various drugs currently in mid to late-stage development. While not a comprehensive review, this paper seeks to extend the thinking on how to treat obesity beyond GLP-1 and into other potentially fruitful areas of research.
What’s It Got To Do With Uric Acid?
There is a clear association between obesity and hyperuricemia (HUA). For example, a large study of Chinese patients1 showed that across the BMI categories, the prevalence of HUA was 5.1% in overweight, 15.2% in obesity I, 16.9% in obesity II, and 32.5% in obesity III. Correlation analysis showed that HUA is strongly correlated with BMI, waist circumference (WC), and hip circumference (HC). The National Health and Nutrition Examination Survey (NHANES) of a US population revealed that overweight and obese individuals had higher uric acid levels than those with the normal BMI, and this also correlated with dietary energy intake2. Of course, this observation is subject to the standard caveat that correlation does not necessarily imply causation.
Uric acid is a product of purine metabolism that is present in human blood and urine. Uric acid has been traditionally considered as a waste product that needs to be eliminated from the body, and an excess is associated with gout and other conditions, but recent studies have shown that it has important roles as a metabolic signaling molecule. Uric acid can act as a danger signal and a modulator of various physiological processes, such as inflammation, oxidative stress, insulin resistance, and cardiovascular function.
Uric acid levels are routinely measured in clinical practice as they can reflect the nutritional status and metabolic health of an individual. Uric acid levels are influenced by dietary intake of purine-rich foods, such as animal proteins, as well as by endogenous production and excretion. High levels of uric acid, or hyperuricemia, can indicate an increased risk of gout, nephrolithiasis, and other metabolic disorders, such as obesity, metabolic syndrome, and type 2 diabetes mellitus3. On the other hand, low levels of uric acid, or hypouricemia, can be associated with neurological diseases, such as Parkinson's disease and multiple sclerosis4.
As a danger signal, uric acid can trigger inflammatory responses and immune activation in response to cellular stress and damage. Uric acid is released from dying cells, where it can activate the innate and adaptive immune system via toll-like receptors, NLRP3 inflammasome, and dendritic cells5. Uric acid can also induce a foraging response and increase food intake in situations of starvation or hibernation³. However, excessive or chronic inflammation caused by uric acid can be harmful and contribute to tissue injury and disease progression6.
As a modulator, uric acid can affect the function and signaling of various cells and organs, such as endothelial cells, smooth muscle cells, adipocytes, pancreatic beta cells, and cardiomyocytes⁵. Uric acid can influence the production and availability of nitric oxide, reactive oxygen species, and cytokines, which are involved in vascular tone, oxidative stress, and inflammation⁵. Uric acid can also interfere with insulin signaling and glucose uptake, leading to insulin resistance and impaired glucose metabolism7.
Uric acid is the terminal product of the purine metabolic pathways. Over the past century, these pathways have come to be recognized not only as fundamental elements of bioenergetics, but also in their role in chemical transmission. Today, it is accepted that deregulation and malfunction of the purine/purinergic system contributes to the pathophysiology of numerous diseases, including gout, diabetes, neurological disease, osteoporosis and cancer.
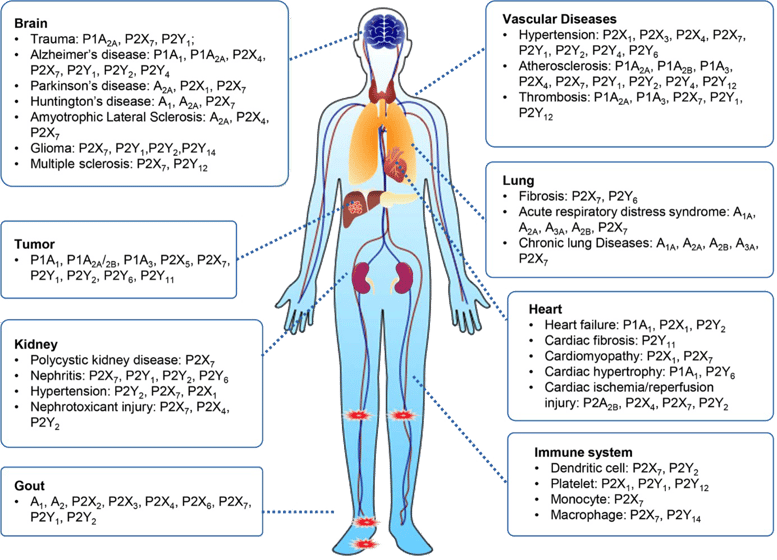
The causative role of purinergic signaling in human diseases. Purinoceptors, including P1, P2X and P2Y receptors, are diffusely expressed in every human body part, such as the nervous system, circulatory system, respiratory system, immune system, and urinary system, among others. Dysregulation of purinoceptor function leads to various diseases, including neurological, rheumatic, cardiovascular, cancer diseases and so on6.
Given this complex metabolic and signaling network, it should come as no surprise that uric acid is also a key player in metabolic syndrome and obesity8. A 2016 article titled “Uric acid in metabolic syndrome: From an innocent bystander to a central player”, expresses the point succinctly.
A More Prominent Role for Uric Acid?
The abstract of that 2016 publication is worth quoting:
"Several experimental and clinical studies support a role for uric acid as a contributory causal factor in these conditions. Here we discuss some of the major mechanisms linking uric acid to metabolic and cardiovascular diseases. At this time the key to understanding the importance of uric acid in these diseases will be the conduct of large clinical trials in which the effect of lowering uric acid on hard clinical outcomes is assessed. Elevated uric acid may turn out to be one of the more important remediable risk factors for metabolic and cardiovascular diseases."
The paper discusses that an elevated serum uric acid is also a potent predictor for the development of obesity9 (a finding originally published in 2003 and based on earlier observations). The paper concludes:
“While uric acid was once the lonely dinner conversation for those suffering from gout or kidney stones, it is now being evaluated as a potential master conductor in the worldwide symphony of obesity, diabetes, and cardiorenal disease”.
A recent meta-analysis addresses the association between metabolic syndrome and uric acid10. It concludes that assessment of uric acid concentration could provide a new avenue for early diagnosis of metabolic syndrome, as a new biomarker and the possibility of new therapeutic targets. Overall, the results of the meta-analysis have shown that people with metabolic syndrome have 8.2% more uric acid, leading the authors to speculate that reducing urate levels could positively impact the development of this syndrome. The evidence that interventions that reduce the levels of uric acid can produce clinically useful weight loss is far from established. Interestingly however, a pilot clinical study11 showed that allopurinol, a standard drug treatment for gout and an inhibitor of xanthine oxidase that lowers serum urate levels, led to a reduction in weight and blood pressure even though there was no caloric acid restriction. As a cheap generic, there is no commercial incentive to investigate whether allopurinol or other urate lowering agents could play a part in treating obesity, but the mechanism deserves further consideration.
Serum uric acid levels depend in large part on intake of high-purine foods and beverages (e.g., meat, beer) and on the intake of dietary fructose which is metabolized to uric acid. High consumption of sugary drinks that contain high fructose corn syrup (HFCS) is known to be associated with high levels of obesity12,13,14. Few would argue that stopping the intake of sodas might be beneficial both for normalization of uric acid and weight loss or maintenance of weight loss.
Uric Acid and Evolutionary Survival
A fascinating observation is that most mammals have low serum uric acid levels (1-2mg/dl) due to the presence of uricase which metabolizes it15. However, uricase activity was progressively attenuated (25 to 15 million years ago) and then silenced about 15 million years ago in apes and prehominids16. There is increasing evidence that the mutation may have provided survival advantages to the ancestral apes at the time, by increasing their ability to store fat in response to a decrease in food (fruit) availability that resulted from global cooling17,18. Raised uric acid stimulates foraging behavior and impulsivity19. Importantly, as fruits and berries ripen, the level of fructose increases and the fruit becomes sweeter, and this leads to increased uric acid levels which in turn leads to elevated gluconeogenesis, higher blood pressure and increased fat deposition20. There is a direct line between increased fructose in Autumn fruits to increased urate - it is a signal to “prepare for winter” by laying down reserves. With today’s Western diets, the high uric acid levels are signaling our bodies to prepare for winter – but winter never comes. A diet of burgers, beer and HFCS-containing carbonated drinks feeds a vicious cycle that results in ever increasing levels of fat deposition
This line of thinking has been popularized by David Perlmutter, MD (https://drperlmutter.com/) whose book, “Drop Acid” makes for compelling reading. It also leads to the idea that allopurinol, as well as quercetin, could be tried as anti-obesity agents. Quercetin is a flavonoid available as a health supplement that has demonstrated anti-hyperuricemic activity through several mechanisms including inhibiting corresponding enzymes in urate production namely xanthine oxidase, adenosine deaminase, and ketohexokinase and increasing renal urate excretion by promoting the activity of urate excretion transporters and suppressing the activity of urate reabsorption transporters21. It’s worth noting though, that there is no widespread belief in this proposition. It also suggests that reducing intake of HFCS and beer, except for some purine-free brands that have become available in Japan, might be beneficial. Although one hardly needs a literature review to reach that conclusion.
The Role of Inflammation
It is well known that weight loss is associated with a reduction of CRP, a standard inflammatory marker22. Uric acid activates the NLRP-3 inflammasome23, which may be an evolutionary mechanism whereby overused muscles produce uric acid and though this signal heightens nociception and triggers behavior to stop use of the relevant muscles. However, urate is far from the only actor; obesity shares with most chronic diseases the presence of an inflammatory component, which accounts for the development of metabolic disease and other associated health alterations. This inflammatory state is reflected in increased circulating levels of pro-inflammatory proteins24. There are a number of plausible causes: (1) an increase in the production of leptin (pro-inflammatory) and the reduction in adiponectin (anti-inflammatory) seem to affect the activation of immune cells; (2) free fatty acids can induce inflammation through various mechanisms (such as modulation of adipokine production or activation of Toll-like receptors); (3) nutrient excess and adipocyte expansion trigger endoplasmic reticulum stress; and (4) hypoxia occurring in hypertrophied adipose tissue stimulating the expression of inflammatory genes and activating immune cells24. The gut microbiome is also likely involved25. Obesity and metabolic disease can also influence the immune system26,27,28,29 and a recent study showed that obesity converted the classical type 2 T helper (TH2)-predominant disease associated with atopic dermatitis to a more severe disease with prominent TH17 inflammation30. Nevertheless, a very recent study31 suggests that in obese mice consuming a high-fat diet, brain-penetrant NLRP3 inhibition and the resulting anti-inflammatory effect confers not only reversal of obesity but metabolic benefits that extend well beyond this. indeed, the level of weight loss in the experimental animals exceeded that produced by GLP-1 treatment.
Effects on the Brain and Behavior
Inflammation predicts decision making characterized by impulsivity, present focus, and an inability to delay gratification32 - at least according to a theoretical model. There is data linking inflammation to eating disorders33, ADHD34 and bipolar disorder35. Moreover, studies indicate that inflammation decreases functional connectivity between the prefrontal cortex (PFC) and striatum and the amygdala in a manner that predicts reward deficits, anhedonia and psychomotor slowing36. A systematic review found a significant alteration of neural circuits primarily involving the frontal and limbic regions; functional activity results showed BMI-dependent hypoactivity of frontal regions during cognitive inhibition and either increased or decreased patterns of activity in several other brain regions, according to their respective role in inhibition processes. The presence of binge eating disorder results in further aggravation of those neural alterations. Connectivity results mainly report strengthened connectivity patterns across frontal, parietal, and limbic networks. Neuroimaging studies suggest significant impairment of various neural circuits involved in inhibition processes in individuals with obesity37.
Put simplistically, under inflammatory conditions the normal higher functions of the PFC in restraining the response of the amygdala leads to greater impulsivity and poorer decision making. When decision-making becomes impaired, individuals may struggle to make safe and rational decisions. Studies show that individuals are more likely to make riskier decisions when the amygdala does not trigger somatic responses or reactions to external stimuli like fear. Impaired decision-making can also cause impulsive behavior, which can sometimes put a survivor at risk for harm. As Dr. Perlmutter puts it in pop science terms, “the PFC is the adult in the room and the amygdala is the teenager.” In his view, excess fructose, meat and beer leads to elevated uric acid which prompts not only metabolic changes that lead to obesity but also to inflammation which hands over brain function to the amygdala and results in even less self-restraint in harmful obesity-inducing behaviors.
This link between inflammation and brain functioning is rarely outlined. This is another argument to consider obesity as a chronic disease that needs to be treated: the vicious circle of inflammation modifying the behavior of obese subjects leading to more eating disorders, and further aggravating obesity.
The Mainstream Pipeline
The current obesity drug pipeline includes over 115 clinical-stage assets under investigation. In recent years, the landscape of gut hormone-based pharmacotherapies for obesity and type 2 diabetes has undergone a rapid evolution. One notable advancement lies in the development of combinations involving GLP1 with other gut hormones such as GIP, glucagon, and amylin, either as dual or triple agonists. These innovative drugs hold the potential for enhanced efficacy in promoting weight loss and addressing obesity-related complications. The recent approval of tirzepatide, a GLP1 and GIP agonist, marks a significant milestone, hinting at the possibility of pharmacotherapy achieving weight loss outcomes akin to those seen with bariatric surgery.
Obesity pipeline by phase, target and route of administration
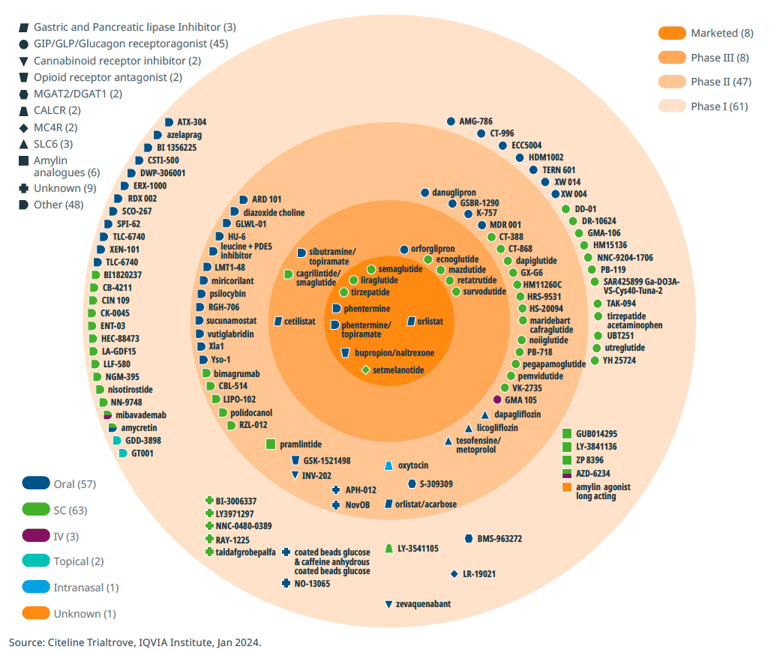
Beyond GLP-1 agonists, the obesity drug landscape encompasses a diverse array of mechanisms, including SGLT2 inhibitors, MGAT2 inhibitors, MC4R agonists, PDE5 inhibitors, PYY analogues, CB1 antagonists, and insulin receptor modulators. Moreover, combination therapies represent another promising avenue of research, suggesting synergistic benefits in combating obesity.
There are several reviews of current and pipeline products38,39,40 and these will not be elaborated further here. Notably, the emergence of new drug classes, exemplified by the cardiovascular benefits of semaglutide demonstrated in a positive cardiovascular outcomes trial (SELECT, with >17,000 participants), has raised the bar for obesity therapeutics. There is still considerable room for improvement in incretin therapies and dual or triple agonists/antagonists, particularly in addressing issues such as gastrointestinal tolerability, the injectable nature of current treatments, and the durability and composition of weight loss (fat vs. lean body mass). Furthermore, exploring strategies for maintaining stable body weight post-treatment or developing novel approaches for weight maintenance represents another important unmet need in obesity management and area of interest.
Concluding Comments
There is an intricate relationship between obesity — especially visceral obesity, as indicated by waist circumference — and hyperuricemia, which can exacerbate complications associated with obesity. Hyperuricemia's role in inflammation amplifies cardiovascular risks and compounds the burden of obesity-related conditions. Understanding the vicious circle of inflammation and how it alters the behavior of obese individuals, thereby worsening eating disorders and aggravating obesity, is essential. Moreover, advocating for straightforward interventions like reducing soda consumption can aid in mitigating hyperuricemia and its related health risks.
Now that practitioners have at their disposal highly effective tools for treating individuals with obesity, the acceptance of obesity as a chronic disease — requiring lifelong treatment similarly to hypertension or hypercholesterolemia — remains hindered by several barriers. These include the budgetary constraints faced by payers and the reluctance of healthcare systems to allocate resources for the treatment of the vast population affected by obesity and its associated complications. These complications extend beyond cardiovascular issues to encompass conditions such as diabetic kidney disease, osteoarthritis, and sleep apnea.
The development of oral small molecules targeting the same pathways as existing peptide-based therapies holds promise for overcoming barriers related to treatment acceptability and cost-effectiveness. Small molecules may offer advantages in terms of ease of administration and production costs, potentially making obesity treatment more accessible to a broader population.
Healthcare systems may agree to reimburse obesity treatments if ongoing cardiovascular outcomes studies deliver positive results. Novo Nordisk's SELECT trial published in 2023, set a high standard by demonstrating that Wegovy-induced weight loss led to a statistically significant 20% reduction in a composite endpoint comprising cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke among overweight or obese patients with prior cardiovascular disease. While the SELECT trial exceeded expectations, it also underscored the importance of conducting further outcomes trials to elucidate additional potential benefits. Competitors in the field must strive to match or surpass Wegovy's performance. Numerous cardiovascular outcome trials (CVOTs), including the SURMOUNT-MMO (tirzepatide in obesity) trial and SURPASS-CVOT (tirzepatide in diabetes), are currently underway.
Obesity is clearly a multifactorial condition, or set of conditions, involving multiple interlocking systems each of which display a high level of complexity. We are absolutely only scratching the surface, and frankly, it is remarkable that we have any effective and apparently safe pharmacological approaches at all. At the same time, there are many different directions for future research well beyond GLP-1 drugs, and it’s certainly clear that the science of obesity is set for major leaps forward in the years ahead.
About the Author:
-
- Anthony Walker, PhD, Managing Partner, Alacrita Consulting
Anthony leverages more than 35 years of experience, including over a decade spent building & managing a biotechnology company and over 20 years as a management consultant to the pharmaceutical & biotech industries. - Special thanks to Dr. Christine McCarthy, Alacrita Partner, for critical review and invaluable suggestions.
- Anthony Walker, PhD, Managing Partner, Alacrita Consulting
Bibliography
- 1 https://doi.org/10.3389/fpubh.2022.986954
- 2 https://doi.org/10.1186/s12902-022-01112-5
- 3 https://doi.org/10.1007/978-3-030-81304-8_7-1
- 4 https://doi.org/10.1038/s41392-021-00553-z
- 5 https://doi:10.1016/j.semnephrol.2011.08.002
- 6 https://doi:10.1161/ATVBAHA.117.309128
- 7 https://doi.org/10.3389/fphar.2020.582680
- 8 https://doi:10.1016/j.ejim.2015.11.026
- 9 https://doi:10.1161/01.HYP.0000091371.53502.D3
- 10 https://doi.org/10.1038/s41598-022-22025-2
- 11 https://doi:10.1016/j.jash.2015.07.008
- 12 https://doi:10.2337/dc10-1079
- 13 https://doi:10.3945/ajcn.113.058362
- 14 https://doi:10.1093/ajcn/86.4.899
- 15 https://doi:10.1093/ajcn/86.4.899
- 16 Kahn K, Serfozo P, Tipton PA. Identification of the true product of the urate oxidase reaction. J Am Chem Soc. 1997; 119(23):5435–5442.
- 17 https://doi:10.1073/pnas.1320393111
- 18 Johnson RJ, Andrews P. Fructose, uricase, and the back-to-Africa hypothesis. Evol Anthropol. 2010; 19:250–257
- 19 Johnson RJ, Andrews P. The fat gene: a genetic mutation in prehistoric apes may underlie today's pandemic of obesity and diabetes. Sci Am. 2015; 313:64–69.
- 20 https://doi:10.1016/j.biopsych.2013.02.024
- 21 https://doi:10.1016/j.jsps.2022.04.013
- 22 https://doi:10.1001/archinte.167.1.31
- 23 http://dx.doi.org/10.1177/1744806919858797
- 24 de Heredia FP, Gómez-Martínez S, Marcos A. Obesity, inflammation and the immune system. Proceedings of the Nutrition Society. 2012;71(2):332-338. doi:10.1017/S0029665112000092
- 25 https://doi.org/10.3390/diseases11010007
- 26 https://doi:10.1016/j.cell.2017.04.004
- 27 https://doi:10.1016/j.cmet.2016.08.016
- 28 https://doi:10.1146/annurev-immunol-042617-053019
- 29 https://doi:10.1038/s41590-018-0251-7
- 30 https://doi.org/10.1038/s41586-022-04536-0
- 31 https://doi:10.1124/jpet.123.002013
- 32 https://doi.org/10.1038/s41598-019-41437-1
- 33 https://doi.org/10.3389/fphar.2022.846172
- 34 https://doi.org/10.1159/000489635
- 35 https://doi.org/10.1186/s12929-021-00742-6
- 36 Ravi M, Miller AH, Michopoulos V. The immunology of stress and the impact of inflammation on the brain and behaviour. BJPsych Advances. 2021;27(3):158-165. doi:10.1192/bja.2020.82
- 37 https://doi.org/10.3389/fnut.2021.609012
- 38 https://doi.org/10.1016/j.eclinm.2023.101882
- 39 https://do.org/10.7759/cureus.46623
- 40 https://doi.org/10.1007/s13300-020-00816-y

